Technical Support
Intended Use
Erwinia amylovora (Fire blight) is a serious contagious bacterial disease of some rosaceous plants (apples, pears and
related trees and shrubs). The bacterium causes the disease Fire blight and is a major threat to the horticultural
industry. Erwinia amylovora or Fire blight is a quarantine disease in Europe and many other countries.
Erwinia amylovora features on the European and Mediterranean Plant Protection Organisation (EPPO) A2 list of pests
recommended for regulation as quarantine pathogens.
Bees and other insects, birds, rain and wind can transmit the bacterium to susceptible tissue. Injured tissue is also
highly susceptible to infection, including punctures and tears caused by plant-sucking or biting insects
Erwinia amylovora Pocket Diagnostic is a rapid diagnostic test designed to be used in the field, nursery or glasshouse.
The test can be performed within minutes by operators with little or no training. Samples for testing can be taken from
any part of the plant, at any stage during the production cycle. Pocket Diagnostic test results form an important part
of sound decision making in routine screening or disease control situations.
Erwinia amylovora Test Kit Contents:
· Test device (x4)
· Bottle of extraction buffer (including ball bearing) (x4)
· Pipette (x4)
· Instructions for Use
Quality Standards
Pocket Diagnostic tests are manufactured in environmentally-controlled facilities by Forsite Diagnostics Ltd, trading
as Abingdon Health, following procedures which meet ISO9001 quality standards and where possible validated to the
European and Mediterranean Plant Protection Organisation (EPPO) standards.
Storage and Shelf life
Pocket Diagnostic Kits should be stored at room temperature (do not exceed 40°C). Tests are supplied with at least 6
months shelf life.
Safety
Pocket Diagnostic test devices contain no hazardous materials to humans, animals, plants or crops. The
accompanying extraction buffer contains a very small amount of sodium azide (0.05%) as a preservative – avoid
ingestion and skin contact. Refer to your government guidelines for the disposal of sodium azide.
Disposal of used tests
In cases where positive or suspect positive results are obtained for quarantine or notifiable pathogens, all kit
components should be regarded as contaminated and should be disposed of appropriately. Contact the Food and
Environment Research Agency (FERA) for further details on how to do this. If you are not based in the UK please
contact your government plant pathology and plant pathogen quarantine department.
Test Procedure
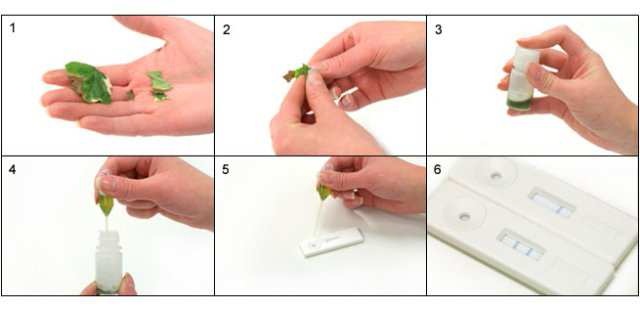
1. Select sample. Where possible select a part of the plant where diseased and healthy material meet, do not use
dead material. Use approximately 25mm square of material (See picture 1). Take care to avoid cross
contamination between samples from hands or cutting tools.
2. Cut or tear sample into small pieces and put into bottle.
3. Shake firmly for 30 seconds (longer for more resistant samples)
4. Wait 30 seconds allow the solution to settle and draw liquid into pipette avoiding too much plant material.
5. Add 2-3 drops into sample well of test strip.
6. Read result after 5-10 minutes.
Interpreting results
A line present next to the T (test line) indicates a positive result.
If the C line (control) does not appear the test has failed and must be repeated.
Ignore any changes to the result which occur after the 10 minutes has elapsed.






